热解合成芳香腈Valorization of Waste PET: Understanding the Role of Active Ammonia in Facilitating PET Depolymerization and Aromatic Nitrile Formation
Valorization of Waste PET: Understanding the Role of Active Ammonia in Facilitating PET Depolymerization and Aromatic Nitrile Formation
Recently, Mr Geliang Xie supervised by assoc. Prof. Lujiang Xu published a research article in Journal of Cleaner Production about preparation of aromatic nitrile by pyrolysis of polyester plastic PET under active ammonia atmosphere.
Realizing the recovery and valorization of waste polyethylene terephthalate (PET) plastics into value-added products have gained significant importance. This study successfully synthesized aromatic nitriles, specifically terephthalonitrile (TPN), from waste polyethylene terephthalate (PET) plastic through in situ catalytic pyrolysis with urea and γ-Al2O3 catalyst. Based on distributed activation energy model, positive interaction between waste PET and urea is responsible for improved thermal decomposition kinetics. The dosage of urea and pyrolysis temperature significantly influenced TPN production. TPN yield reached 32.5% with selectivity of 85% at 550°C and equivalent urea dosage. Density functional theory calculations validated the role of active ammonia in reducing energy barriers and thus facilitating PET depolymerization. The study highlights the feasibility of recycling waste PET into valuable aromatic nitriles through in situ catalytic pyrolysis with urea and enhances the understanding of the pyrolysis process.
Publication:
GL Xie, GQ Zhu, YF Kang, MX Zhu, QQ Lu, C He, LJ Xu*, Zhen Fang, Valorization of Waste PET: Understanding the Role of Active Ammonia in Facilitating PET Depolymerization and Aromatic Nitrile Formation. Journal of Cleaner Production (IF 11.1), 434 (2024), 140204. https://doi.org/10.1016/j.jclepro.2023.140204.
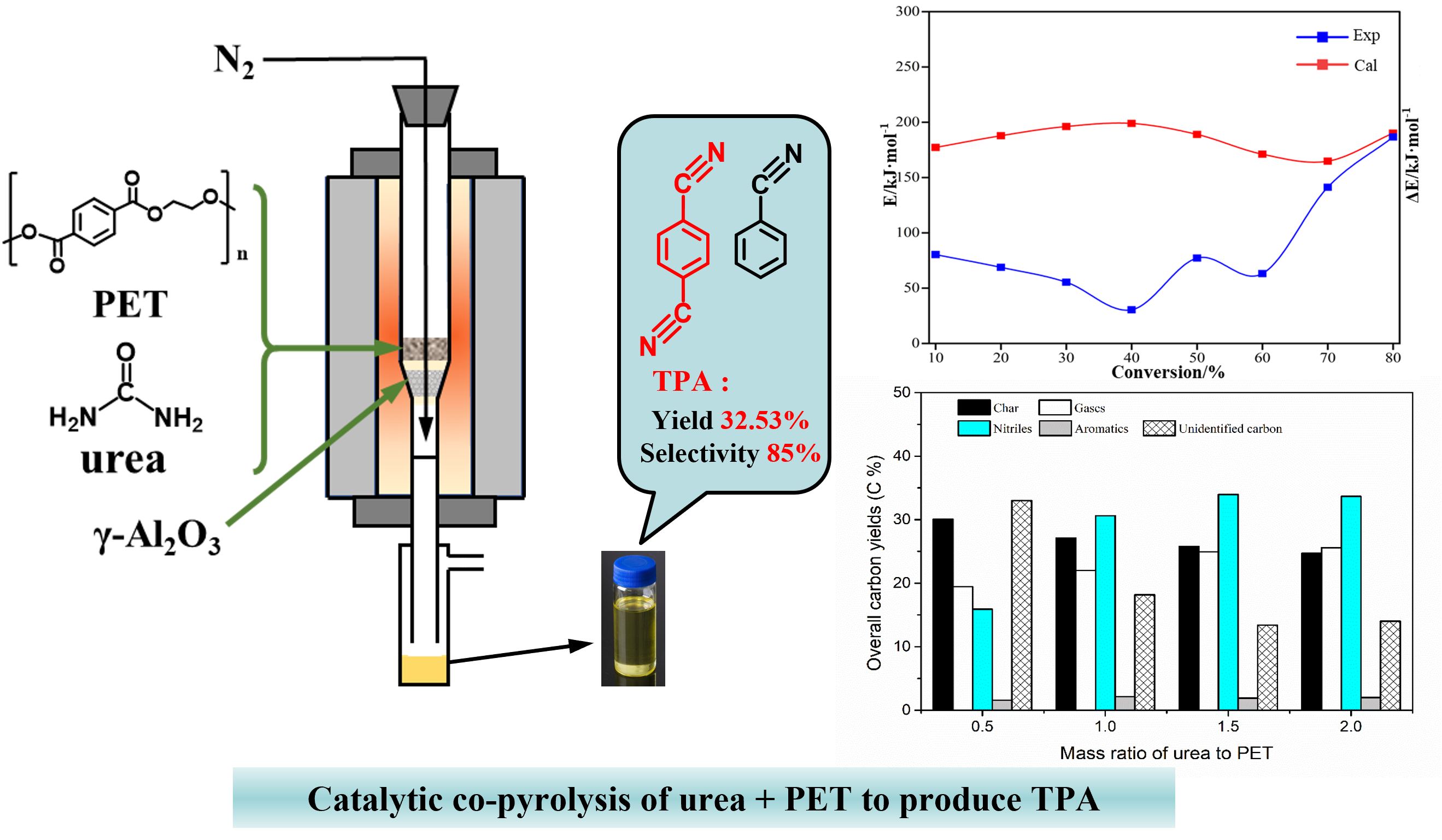
With urea as the ammonia source and γ-Al2O3 as the catalyst, terephthalonitrile (TPA) can be synthesized by catalytic co-pyrolysis of PET. Co-pyrolysis reduces the activation energy by 50%. The yield and selectivity of TPN under optimal conditions were 32.53% and 85%, respectively.Density functional theory (DFT) calculations revealed the possible mechanism of PET formation of TPN in the presence of urea and γ-Al2O3.
以尿素为氨源,PET在γ-Al2O3催化剂下进行催化共热解可以合成对苯二甲腈(TPA)。共热解可使活化能降低50%,最佳条件下TPN的收率和选择性分别为32.53%和85%。密度泛函理论(DFT)计算揭示了在尿素和γ-Al2O3存在下PET形成TPN的可能机理。
硕士生谢葛亮在徐禄江副教授指导下,在国际学术期刊Journal of Cleaner Production上发表研究性论文:
废PET的增值:了解活性氨在促进PET解聚和芳腈生成中的作用
最近,硕士生谢葛亮在徐禄江副教授指导下,在国际学术期刊Journal of Cleaner Production (Q1; Impact factor: 11.1)上发表了一篇关于活性氨氛围下热解聚酯塑料PET制备芳香腈的研究性论文。
实现废旧聚对苯二甲酸乙二醇酯(PET)塑料的回收和增值为增值产品具有重要意义。以聚对苯二甲酸乙二醇酯(PET)废塑料为原料,在尿素和γ-Al2O3催化剂的催化下原位热解合成了芳香族腈,特别是对苯二甲酸乙二醇酯(PET)。基于分布活化能模型,废pet与尿素的正相互作用是改善热分解动力学的原因。尿素用量和热解温度对TPN产量有显著影响。在550℃、同等尿素用量条件下,TPN收率为32.5%,选择性为85%。密度泛函理论计算验证了活性氨在降低能垒从而促进PET解聚中的作用。该研究突出了利用尿素原位催化热解将废PET回收为有价芳香烃的可行性,并加深了对热解过程的认识。
详情可见:
GL Xie, GQ Zhu, YF Kang, MX Zhu, QQ Lu, C He, LJ Xu*, Zhen Fang, Valorization of Waste PET: Understanding the Role of Active Ammonia in Facilitating PET Depolymerization and Aromatic Nitrile Formation. Journal of Cleaner Production (IF 11.1), 434 (2024), 140204. https://doi.org/10.1016/j.jclepro.2023.140204.