Supercritical water gasification (SCWG) of waste cooking oil
30 10 月, 2018Supercritical water gasification (SCWG) of waste cooking oil
Recently, Prof. JA Kozinski group at Waterloo collaborated with Profs. AK Dalai at U Saskatchewan and Zhen FANG studied SCWG of waste cooking oil.
In the work, Dr. S Nanda (U Western Ontario) et al. studied waste cooking oil gasification at variable temperatures (375-675°C), feed concentration (25-40 wt%) and reaction time (15-60 min) to investigate their effects on syngas yield and composition. Maximum yields of hydrogen (5.16 mol/kg) and total gases (10.5 mol/kg) were obtained at optimal temperature, feed concentration and reaction time of 675°C, 25 wt% and 60 min, respectively. At 5 wt% loading, Ru/Al2O3 enhanced hydrogen yield (10.16 mol/kg) through water-gas shift reaction, whereas Ni/Si-Al2O3 improved methane yield (8.15 mol/kg) via methanation reaction. The trend of hydrogen production from catalytic supercritical water gasification of waste cooking oil at 675°C, 25 wt% and 60 min decreased as Ru/Al2O3 > Ni/Si-Al2O3 > K2CO3 > Na2CO3. The results indicate the recycling potential of waste cooking oil for hydrogen production through hydrothermal gasification.
Results were published:
S Nanda, R Rana, H Hunter, Zhen Fang, AK Dalai, JA Kozinski*, Hydrothermal Catalytic Processing of Waste Cooking Oil for Hydrogen-rich Syngas Production, Chemical Engineering Science, https://doi.org/10.1016/j.ces.2018.10.039 (2018).
—————————
超临界水气化(SCWG)废食用油
最近,加拿大滑铁卢大学的JA Kozinski教授研究组与萨斯喀彻温大学AK Dalai教授和方真教授教授合作,研究了超临界水气化废食用油。
S Nanda博士(U Western Ontario)等研究了不同温度(375-675 °C)、物料浓度(25- 40 wt %)和反应时间(15- 60 min)对废食用油气化的影响,研究了它们对合成气产量和组成的影响。在最佳条件下(675 °C, 25 wt %, 60 min)分别获得最大产氢量(5.16 mol/kg)和总气体量(10.5 mol/kg)。在5wt %的催化剂负荷下,Ru/Al2O3通过水煤气变换反应提高了产氢量(10.16 mol/kg),而Ni/Si-Al2O3通过甲烷化反应提高了产氢量(8.15 mol/kg)。在675 °C、25 wt%和60 min时,催化超临界水气化制氢的趋势随着Ru/Al2O3 Ni/Si-Al2O3 > K2CO3 > Na2CO3的趋势下降。结果表明,废弃食用油通过水热气化制氢具有回收利用潜力。
结果发表在:
S Nanda, R Rana, H Hunter, Zhen Fang, AK Dalai, JA Kozinski*, Hydrothermal Catalytic Processing of Waste Cooking Oil for Hydrogen-rich Syngas Production, Chemical Engineering Science, https://doi.org/10.1016/j.ces.2018.10.039 (2018).
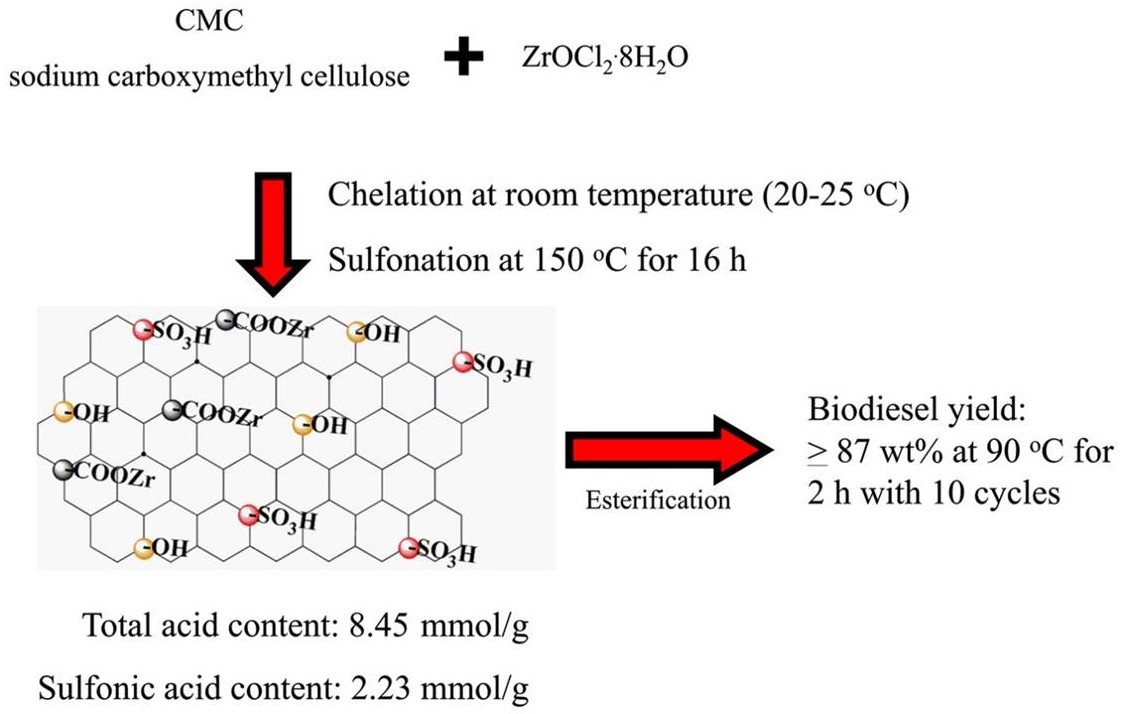
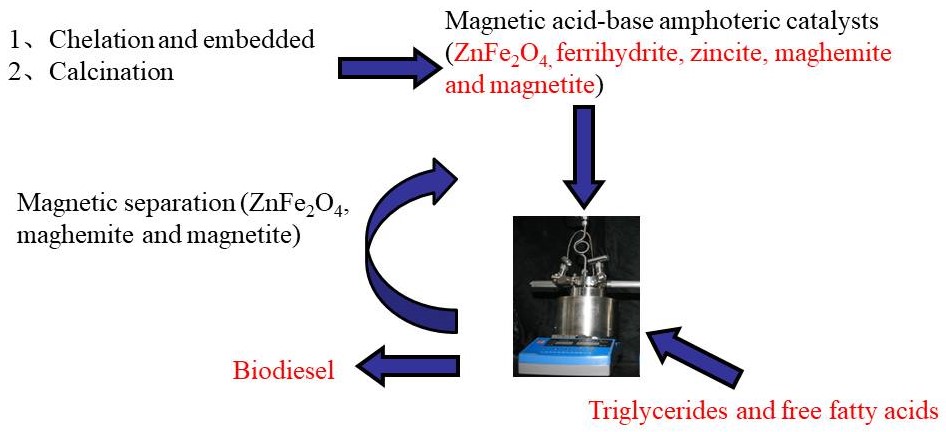 Alkaline oxides concerted with acidic -COOFe structure, for the one-pot esterification and transesterification of high AV Jatropha oils without saponification. Zn8@Fe-C400 achieved nearly 100% Jatropha biodiesel yield at 160 oC within 4 h, and was used for at least 10 cycles with biodiesel yield of >94.3% at AV of 6.3 mg KOH/g.(磁性酸碱两性催化剂通过两步法合成:羧甲基纤维素钠和Fe3+螯合生成的酸性结构-COOFe将碱性氧化物包埋在催化剂的内部,煅烧步骤将部分的-COOFe结构还原成Fe3O4,为催化剂引入磁性。合成的Zn8@Fe-C400可以催化酸值为6.3 mg KOH/g的小桐子油获得100.0%的生物柴油产率,循环使用10次小桐子生物柴油的产率仍然大于94.3%)
Alkaline oxides concerted with acidic -COOFe structure, for the one-pot esterification and transesterification of high AV Jatropha oils without saponification. Zn8@Fe-C400 achieved nearly 100% Jatropha biodiesel yield at 160 oC within 4 h, and was used for at least 10 cycles with biodiesel yield of >94.3% at AV of 6.3 mg KOH/g.(磁性酸碱两性催化剂通过两步法合成:羧甲基纤维素钠和Fe3+螯合生成的酸性结构-COOFe将碱性氧化物包埋在催化剂的内部,煅烧步骤将部分的-COOFe结构还原成Fe3O4,为催化剂引入磁性。合成的Zn8@Fe-C400可以催化酸值为6.3 mg KOH/g的小桐子油获得100.0%的生物柴油产率,循环使用10次小桐子生物柴油的产率仍然大于94.3%)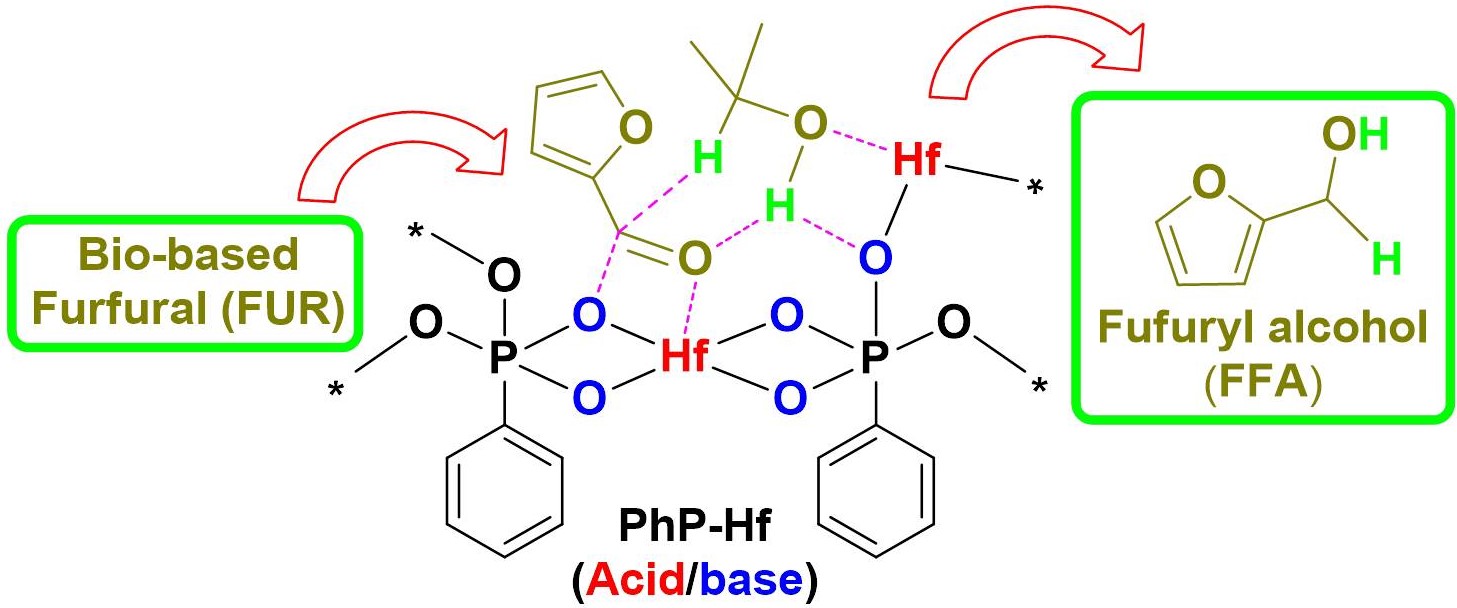 Mesoporous PhP-Hf(1:1.5) nanohybrid material bearing acidic and basic sites promotes catalytic transfer hydrogenation of furfural to furfuryl alcohol with 97.6% yields with 2-propanol as solvent and hydrogen donor source at 120 °C in 2 h reaction time.【介孔PhP-Hf(苯磷酸-铪)纳米MOF(金属有机框架材料)复合物同时具有酸性和碱性位点,促进了糠醛加氢催化转化为糠醇,120℃反应两小时,使用异丙醇作为反应溶剂和氢供体时,糠醇产率为97.6%。】
Mesoporous PhP-Hf(1:1.5) nanohybrid material bearing acidic and basic sites promotes catalytic transfer hydrogenation of furfural to furfuryl alcohol with 97.6% yields with 2-propanol as solvent and hydrogen donor source at 120 °C in 2 h reaction time.【介孔PhP-Hf(苯磷酸-铪)纳米MOF(金属有机框架材料)复合物同时具有酸性和碱性位点,促进了糠醛加氢催化转化为糠醇,120℃反应两小时,使用异丙醇作为反应溶剂和氢供体时,糠醇产率为97.6%。】