生物质电极Regulation of biochar interfaces by loading an Fe,N-carbon layer for the oxygen reduction reaction in acid electrolytes
10 1 月, 2026Regulation of biochar interfaces by loading an Fe,N-carbon layer for the oxygen reduction reaction in acid electrolytes
Recently, under the supervision of Professor Zhen Fang, PhD student Xiao-ru Meng published a research article in Applied Surface Science focusing on the interfacial engineering of biochar-supported iron-based catalysts for the oxygen reduction reaction in acidic media.
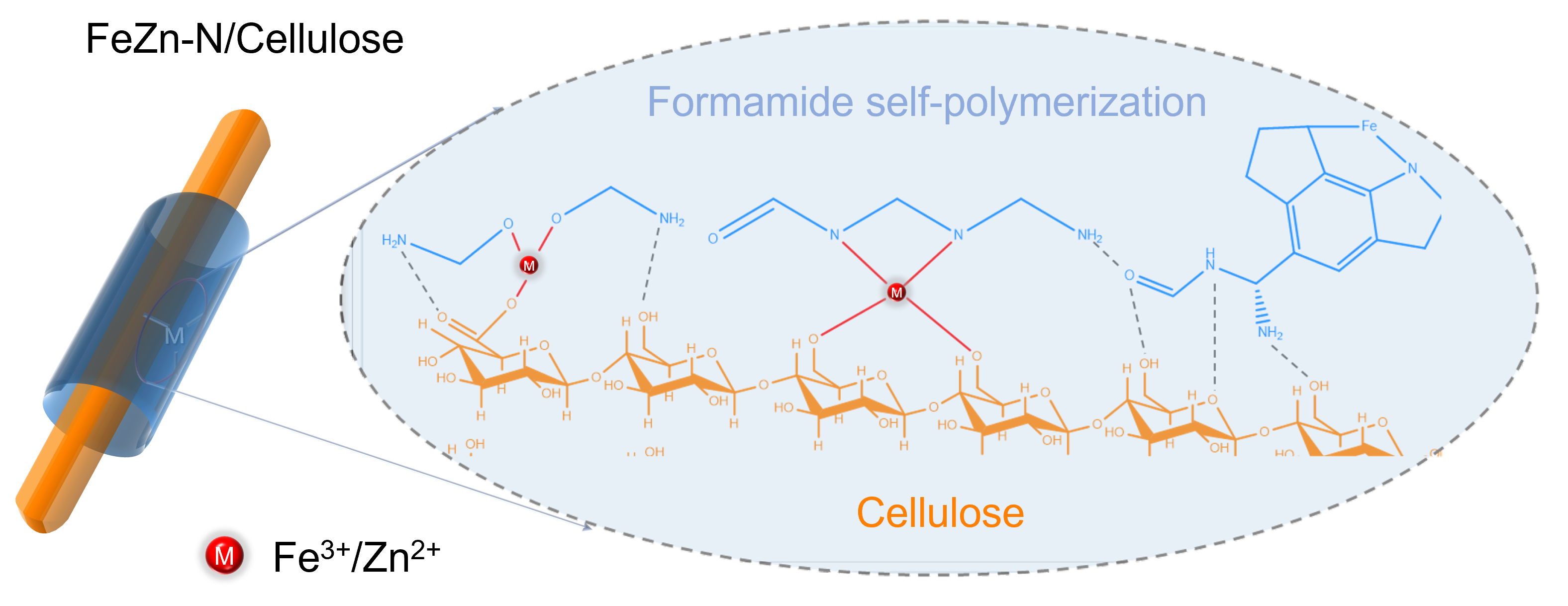
Oxygen reduction catalyst (Fe/Fe3N/C) with Fe/Fe3N encapsulated carbon active sites were synthesized by coated Fe, N layer onto cellulose-derived carbon through the coordination of iron (Fe3+) and formamide solution with cellulose and subsequent carbonization to enhance electrical conductivity of biochar surfaces as cathode in fuel cells. Fe/Fe3N/C catalyst exhibited a half-wave potential of 0.74 V vs. reversible hydrogen electrode in 0.5 M H2SO4 with excellent stability over 100 h. Electrochemical impedance spectroscopy tests showed that the charge transfer resistance was only 1.671 Ω using this novel method, indicating that the cellulose-derived carbon in the core significantly enhanced the electron transport efficiency. Quantum mechanical calculations confirmed strong coordination interaction between the carbonyl groups in formamide and the hydroxyl groups in cellulose with Fe to form Fe/Fe3N structure by carbonization. This study provides an effective renewable material solution for Proton exchange membrane fuel cells.
Results were published in Applied Surface Science:
XR Meng, PD Wu, S Gao, Zhen Fang*, Regulation of biochar interfaces by loading an Fe,N-carbon layer for the oxygen reduction reaction in acid electrolytes, Applied Surface Science, 720 (2026), 165304. https://doi.org/10.1016/j.apsusc.2025.165304
Formation of a conductive Fe,N-carbon layer on the surface of cellulose-based carbon nanofibers在纤维素基碳纳米纤维表面构建导电铁氮共掺杂碳层
负载Fe,N-碳层来调控生物炭界面,用于酸性电解质中的氧还原反应
最近,在方真教授的指导下,博士生孟晓茹在《Applied Surface Science》上发表了一篇关于利用生物炭进行界面调控制备生物炭负载铁基催化剂,用于酸性介质中的氧还原反应的研究文章。
通过将铁(Fe³⁺)与甲酰胺溶液在纤维素表面配位并碳化的方法,在纤维素衍生碳表面包覆Fe/N层,成功制备了具有Fe/Fe₃N封装碳活性位点的氧还原催化剂(Fe/Fe₃N/C),旨在提升生物炭表面作为燃料电池阴极的电导率。该Fe/Fe₃N/C催化剂在0.5 M H₂SO₄电解液中相对于可逆氢电极表现出0.74 V的半波电位,并具有超过100小时的卓越稳定性。电化学阻抗谱测试表明,采用该新颖方法制备的催化剂电荷转移电阻仅为1.671 Ω,证实纤维素衍生碳核芯显著提升了电子传输效率。量子力学计算验证了甲酰胺中的羰基与纤维素中的羟基通过碳化过程与铁形成强配位作用,最终构建Fe/Fe₃N结构。本研究为质子交换膜燃料电池提供了一种有效的可再生材料解决方案。
结果发表在Applied Surface Science:
XR Meng, PD Wu, S Gao, Zhen Fang*, Regulation of biochar interfaces by loading an Fe,N-carbon layer for the oxygen reduction reaction in acid electrolytes, Applied Surface Science, 720 (2026), 165304. https://doi.org/10.1016/j.apsusc.2025.165304